
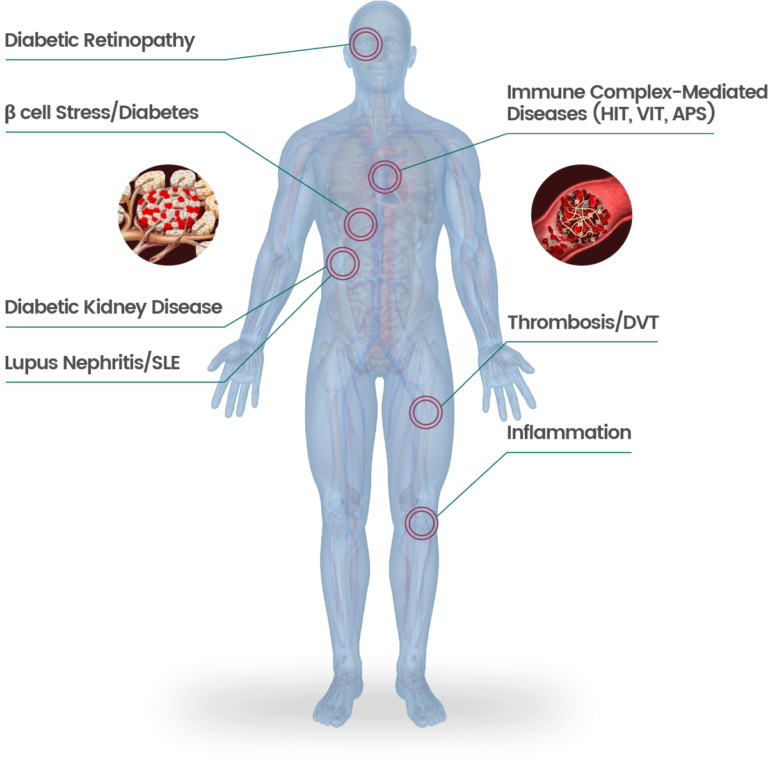
12-Lipoxygenase
12-lipoxygenase (12-LOX) is an enzyme that oxidizes fatty acids, generates proinflammatory metabolites and has been implicated in a myriad of diseases including Heparin-Induced Thrombocytopenia (HIT) and Type 1 Diabetes (T1D). We are developing therapies for these diseases with a clinical program in HIT, preclinical program in T1D and discovery research aimed at expanding our 12-LOX inhibitors to additional diseases.
Learn More about the Target
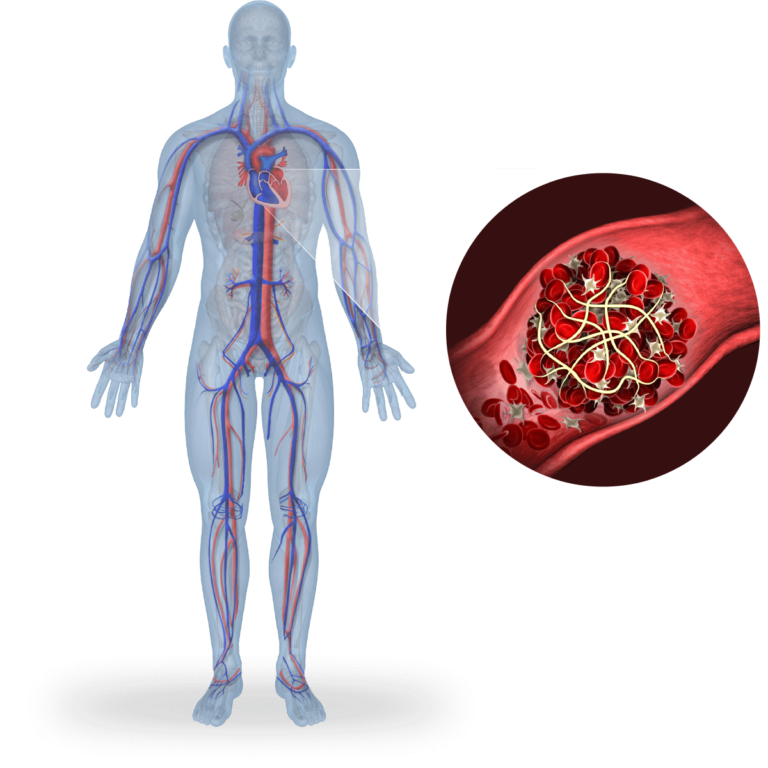
Heparin-Induced Thrombocytopenia (HIT)
HIT is a deadly syndrome characterized by hyperactivated platelets and a devastating pro-thrombotic state. Targeting 12-LOX inhibits the production of 12-HETE, a proinflammatory metabolite known to play a role in platelet activation and thrombosis. We received Orphan Designation and Fast Track Designation for our first-in-class drug product, VLX-1005, addressing the underlying cause of this immune-driven blood clotting.
Learn MoreMeet the Team
Recent News

Veralox Therapeutics Expands Its Pipeline with the Exclusive Option to Acquire Nudge Therapeutics
Exclusive agreement to acquire Nudge Therapeutics includes their series of cGAS inhibitors. Nudge has developed novel potent small molecule cGAS inhibitors that prevents the overproduction of Type 1 interferons which … Read More
Veralox Therapeutics Announces EMA Orphan Drug Designation for VLX-1005
— Receives New Designation for Treatment of Platelet-Activating Anti-Platelet Factor 4 (PF4) Disorders — Complements FDA Orphan Drug Designation and Fast Track Designation for VLX-1005 Frederick, MD, Aug. 14, 2024 … Read More
Veralox Names Jonathan Mow as Chief Executive Officer as the Company Advances Development of First-in-Class Therapies for Immune-Mediated Diseases in Conjunction with Financing
— New Financing of $24 Million Fully Funds the Phase 2 Clinical Program for Lead Candidate VLX- 1005 — Company Prepares to Evaluate VLX-1005 in Heparin-Induced Thrombocytopenia (HIT) FREDERICK, Md., … Read More





















